Parler
Parler Gab
Gab
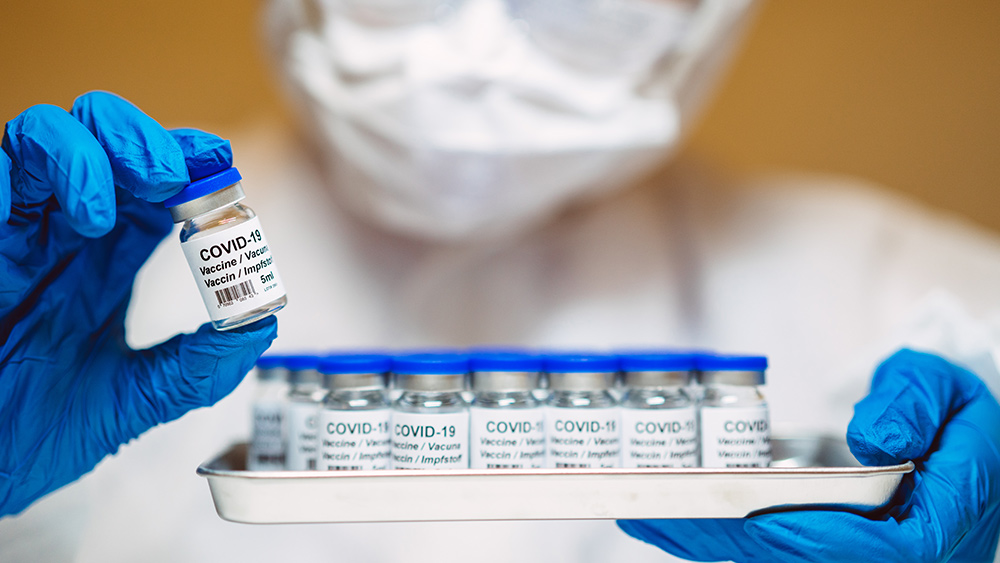
A history of hasty vaccine development
Operation Warp Speed, the Trump administration's flagship initiative during the early stages of the COVID-19 scandal, was lauded for its speed but criticized for its lack of transparency and the potential risks it posed to public health. The rapid development and emergency authorization of mRNA vaccines from Pfizer-BioNTech and Moderna, while credited with saving lives, also caused significant side effects and the long-term impacts of these novel technologies. The ARCT-2304 vaccine, which uses self-replicating mRNA, is the latest in a series of fast-tracked projects that continue to push the boundaries of what is considered safe and ethical in vaccine development.The dangers of self-amplifying mRNA technology
Self-amplifying mRNA vaccines, like ARCT-2304, are designed to replicate within the body's cells, potentially triggering a stronger immune response at lower doses. While this may sound promising, the technology remains largely experimental, and the long-term effects are not yet fully understood. The rapid replication of mRNA within cells could lead to unintended consequences, such as overstimulation of the immune system, which might result in severe adverse reactions. Moreover, the use of self-replicating RNA raises concerns about genetic modification and the potential for unintended genetic changes in the host.The role of BARDA and the Gates Foundation
BARDA's involvement in the ARCT-2304 project is particularly troubling, given its history with Operation Warp Speed. The agency's expansive authority to fund, coordinate, and fast-track pandemic-related therapeutics and vaccines has been criticized for prioritizing speed over safety. The Gates Foundation's commitment of $782,543 to support the project further entrenches the public-private partnership model, which has been a source of controversy due to the potential for conflicts of interest and lack of accountability. The Trump administration's continued support for these experimental technologies, despite the known risks and failures of Operation Warp Speed, is deeply concerning. The Fast Track designation for ARCT-2304 is a clear indication that the administration is more interested in rapid deployment than in ensuring the safety and efficacy of these vaccines. This approach not only puts public health at risk but also undermines trust in the regulatory process. The recent dinner between Bill Gates and President Trump at Mar-a-Lago, where they discussed global health initiatives, further highlights the administration's alignment with private interests. Gates, a long-time advocate for vaccine technologies, has a vested interest in the success of these projects, and his influence on the administration's health policies is evident. The conversation, which lasted about three hours, reportedly focused on the importance of maintaining programs like the President's Emergency Plan for AIDS Relief (PEPFAR) and the potential for accelerated medical innovation. However, this alignment with private interests raises questions about the true motivations behind the administration's vaccine agenda. Sources include: YourNews.com X.com EconomicTimes.comStudy: Daily soda habit linked to 5x higher risk of ORAL CANCER in women
By Ava Grace // Share
Trump administration secures 18 trade proposals, boosting market confidence
By Cassie B. // Share
Musk to step back from DOGE to focus on Tesla amid political backlash
By Cassie B. // Share
By Finn Heartley // Share
“What’s In Your Milk?” The unseen dangers of rBGH and the battle for transparency
By Belle Carter // Share
Legacy of danger: DDT persistence in Canadian trout reveals long-term environmental neglect
By Willow Tohi // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share