Parler
Parler Gab
Gab
Public and private health agencies still recommend Pfizer's Abrysvo to pregnant women despite preterm risks
But despite all this, several private and public health agencies still recommend Pfizer’s Abrysvo for pregnant women. In August 2023, the Food and Drug Administration (FDA) approved Pfizer's Abrysvo for pregnant women to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by RSV in infants from birth through six months of age. (Related: Guinea pigs: Pfizer gets FDA approval to experiment on pregnant women with new RSV vaccine linked to premature birth.) "RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization," said Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research at that time. "This approval provides an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease." Following this, in September 2023, the Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices recommended administering the vaccine to pregnant women between weeks 32 and 36 of their pregnancies to shield newborns from RSV-related lower respiratory tract illness after delivery. Even the American College of Obstetricians and Gynecologists advocates for a single dose of Pfizer's RSV vaccine for expectant mothers in December 2023. VaccineDamage.news has more information on dangerous and experimental new vaccinations. Watch this clip from "Faithful Freedom with Teryn Gregson" on Red Voice Media discussing how the RSV vaccines are the new Wuhan coronavirus (COVID-19) vaccines. This video is from the Red Voice Media channel on Brighteon.com.More related stories:
Pfizer's experimental RSV vaccine under investigation for causing rare nervous system disorder.
FDA panel unanimously supports new RSV antibody drug for infants.
Experts tell RFK, Jr. concerning RSV vaccines: "We have to stop these shots!"
Sources include: ChildrensDefenseHealth.org FDA.govChris Cuomo admits to taking IVERMECTIN for his vaccine injury
By Ava Grace // Share
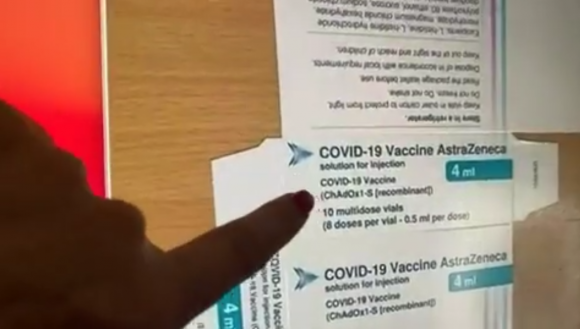
AstraZeneca WITHDRAWS COVID-19 injection worldwide after admitting it causes BLOOD CLOTS
By Ava Grace // Share
Pfizer just agreed to quietly settle 10,000-plus Zantac cancer lawsuits
By Ethan Huff // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share