Parler
Parler Gab
Gab
Habitat loss and overharvesting have affected the 475-million-year-old species in the past, but the massive growth of the pharmaceutical industry seriously threatens the species. The five corporations operating in South Carolina, New Jersey, Massachusetts, Virginia and Maryland reportedly drained the blood of more than 700,000 crabs in 2021, which NPR reported is more than any other year since officials started keeping track in 2004. Larry Niles, a wildlife biologist and leader of the nonprofit Horseshoe Crab Recovery Coalition (HCRC), which unites different organizations into a single concerted effort to end the killing of horseshoe crabs on the Atlantic coast, told The Defender those industry numbers severely undercount the number of harvested crabs. Horseshoe crabs are “a finite source with a potentially infinite demand and those two things are mutually exclusive,” Allen Burgenson, of Swiss biotech Lonza, told Agence France-Presse last year. Lonza produces the LAL test and also has developed a synthetic alternative to horseshoe crab blood. Prioritizing ‘money over the health of the stock’ Horseshoe crabs are one of the oldest species on the planet. They are considered a keystone species, which means many other species depend on the horseshoe crab for survival. Their nutrient-rich eggs are an important food source for migratory birds and marine animals. The rufa red knot, a federally listed shorebird species at risk of extinction, relies on horseshoe crab eggs for food during its annual 9,000-mile migration from Tierra del Fuego at the southern tip of Argentina to its breeding grounds in the Canadian Arctic. But pressures from harvesting crabs for bait, habitat loss and biomedical bleeding have led to a 60% crash in the population along the Atlantic coast over the last 25 years. As a result, shorebird and fishery populations have also crashed. About 94% of red knots have disappeared over the past 40 years. And despite recent harvest regulations, horseshoe crab populations have not increased, according to the HCRC. Niles said part of the problem is that horseshoe crabs are treated as if they have no value — data is not collected on them in any systematic way and they are not protected. He said: “The reality is that the bleeding companies who are extracting the blood and the lysate from the blood are making hundreds of millions of dollars off of the east coast horseshoe crabs because they’re able to hide all the data, including the value. “It perpetuates this myth that the crab is worthless, but in fact, it’s very likely the most valuable fishery on the Atlantic coast. … “Yet the agencies are treating it like it’s valueless.” And given pharma’s resources, it is hard to fight them, Niles said. “We’re up against this system that really prioritizes money over the health of the stock,” he said, adding that along the East Coast, “every fishery is essentially stressed to the breaking point.” Shadowy practices and limited regulation NPR reported that the horseshoe crab industry falls into a regulatory gray area because they aren’t regulated by fisheries or by biomedical regulations — and the regulations that do exist for the treatment of crabs are largely unenforced. There are “best practices” that function as guidelines, but audio recordings of industry meetings obtained by NPR indicate that companies involved in harvesting show little concern for following the non-binding guidelines. Niles said regulations for protecting marine life are much looser than those protecting terrestrial wildlife. For terrestrial animals, he said, corporations have to prove their practices won’t damage the population. But for fisheries, he said, “it’s the opposite. If you think that they are destroying a population, you have to develop the data to prove it.” HCRC has developed its own “best practices” guidelines and says if the practices were implemented, they would actually preserve and re-grow the crab populations. The group is working to get the industry to incorporate them. The horseshoe crab blood extraction industry is dominated by giant multinational firms like the Japanese Fujifilm and Charles River Laboratories, a $22 billion publicly traded pharmaceutical company that provides half of the world’s supply of the horseshoe blood-derived test. In April, a South Carolina District Court issued an injunction against Charles River Laboratories, prohibiting them from harvesting horseshoe crabs during the 2023 season on key red knot migration stopover beaches in South Carolina, in response to a lawsuit brought by the Defenders of Wildlife and other conservation groups against Charles River Laboratories. The injunction also stopped the lab’s practice of keeping crabs in holding ponds prior to bleeding them, where they cannot spawn, so the migratory birds have no access to food. “This settlement is a welcome reprieve for the threatened red knot,” Ben Prater, Defenders of Wildlife Southeast program director, said in a press release. “For years, Charles River Laboratories has overharvested horseshoe crabs, depleting a vital food source for red knots. As these long-distance migrants return to the shores of South Carolina this month we are grateful they now have a better chance to thrive.” But NPR reported that Charles River was responding by simply moving operations elsewhere. It also reported that when it requested annual reports from states where the bleeding was based, the company shared only highly redacted information, which makes it difficult to assess collection and mortality numbers for the crabs. Are high profit margins on horseshoe crab blood hindering progress on synthetic alternative? There is a synthetic alternative, called recombinant factor C (rFC), which can be used to test for endotoxins. The European Pharmacopoeia has confirmed that it is a reliable alternative, but the U.S. Pharmacopoeial Convention (USP) — the nonprofit scientific organization that sets the legally recognized standards for the strength, purity and quality of medicines manufactured and distributed in the U.S. — has not yet approved it. Environmental groups like HCRC and Revive and Restore say the transition to this synthetic alternative is essential for protecting the species. Biologists Jeak L. Ding and Bo Ho, from the National University of Singapore, produced rFC in yeast. They licensed the process to Lonza, which brought it to market as PyroGene. A German company named Hyglos has been working on another synthetic endotoxin detector. Wildlife nonprofit Revive and Restore reports that the patent for rFC was initially withheld from production after it was created because the profit margins on horseshoe crab blood are so high — it sells for approximately $29,000 per quart. Some of the corporations that harvest horseshoe crabs also manufacture and sell the synthetic alternative, but Charles River doesn’t and it continues to lobby to expand its harvest territory, The State reported last year. A 2020 paper by rFC creator Ding and her colleagues said that the continued production and use of the horseshoe crab-harvested LAL has been a barrier to the production of rFC. Others indicate most pharmaceutical companies in the U.S. have not turned to a synthetic alternative because of their reluctance to go through the regulatory process. Regulators like the FDA follow the USP guidelines. Shifting to an alternative method requires additional testing to show the method is equivalent to the existing approved LAL method. A recent op-ed by environmentalist, horseshoe crab expert and author Deborah Cramer in The New York Times faults the USP for repeatedly and inexplicably failing to set standards for the use of rFC in the U.S. The USP has proposed two sets of standards since 2019 but failed to approve them and the committee responsible for the approval was dismissed last year. A new one has been convened. If USP approves standards, drug makers can use the synthetic test without having to go through the lengthy process of winning approval by the FDA, according to Cramer. She wrote that according to Pfizer, a green light from USP would save drugmakers “weeks of laboratory validation tests and documentation for every new product and subsequent F.D.A. regulatory review and approval.” Eli Lilly is currently the only U.S. pharmaceutical company using rFC and it has gone through the FDA regulatory review process. Biotech and biopharmaceutical companies and venture capitalists interviewed for the Times op-ed all indicated they are eager to enter the new market. Read more at: ChildrensHealthDefense.orgThe heart of environmentalism is care for vulnerable plants, animals, people, and places.https://t.co/FDDW2fkBEI
— Robert F. Kennedy Jr (@RobertKennedyJr) June 15, 2023
ALL BARK, NO BITE: Ukraine’s much-hyped counteroffensive SLOWED by vast minefield
By Belle Carter // Share
Study: Regular exercise found to lower risk of developing specific cancer types
By Zoey Sky // Share
Dear House GOP: Stop making excuses and impeach Alejandro Mayorkas immediately
By News Editors // Share
Tucker Carlson: ‘Pride’ is a ‘religious war’ that may bring same fate as Sodom and Gomorrah
By News Editors // Share
Permanent dictator: Zelensky declares NO MORE ELECTIONS in Ukraine until war ends
By Ethan Huff // Share
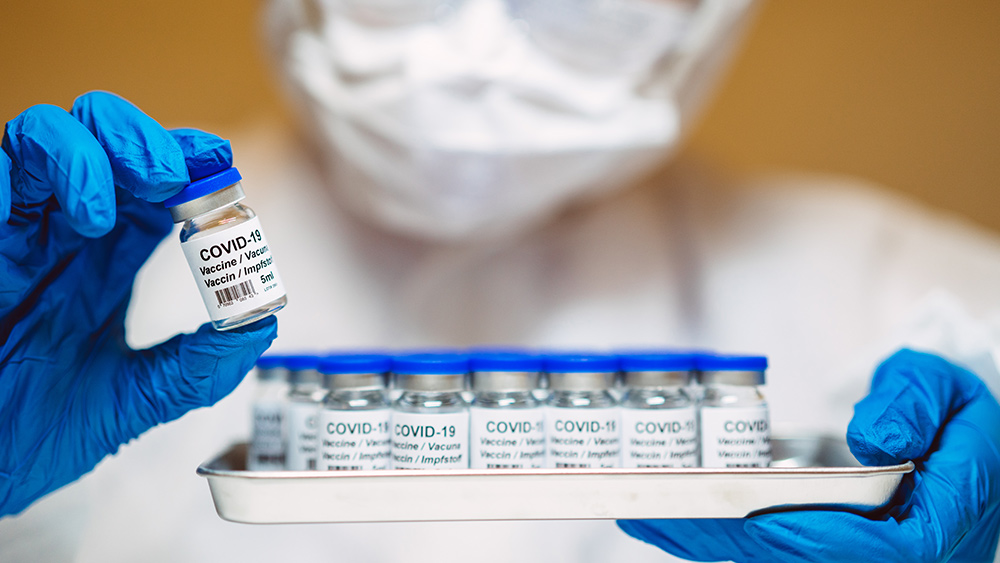
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share